
Disease of the Month - Alport Syndrome
Shots:
- To keep our readers acquainted with several disease conditions, ongoing trials, and available treatment options, PharmaShots brings every month a detailed take on a particular disease after thorough research
- In the next installment to our disease report, this month we have covered a detailed analysis of Alport Syndrome, which is a rare genetic disorder characterized by kidney disease, compromised vision, and hearing loss
- March is observed as Alport Syndrome Awareness Month, an initiative to raise awareness of the genetic disorder
Introduction1,2,3,4
Alport syndrome is a rare hereditary kidney disease that causes genetic alterations to the human’s normal kidney functioning. This syndrome majorly affects the kidney’s glomeruli, the filtering units of the kidney. These genetic alterations in the kidney result in a lack of the production of normal Type IV Collagen Proteins. Moreover, Alport Syndrome may even cause hearing and vision abnormalities. It occurs due to the mutations in the COL4A3, COL4A4, and COL4A5 genes, which are responsible for encoding different parts of the connective tissue protein Type IV Collagen
Alport Syndrome can be categorized under three genetic types, including
- X-linked Alport syndrome (XLAS): It is the most prevalent type of Alport Syndrome with around 80% of patients having the X-linked variant. By the age of 40, 90% of X-linked Alport syndrome males experience renal failure if they are left untreated. Kidney failure affects women less commonly and slower than men.
- Autosomal Recessive Alport syndrome (ARAS): This variant of Alport Syndrome occurs when an abnormal gene is carried by both parents and is passed on to the offspring. The autosomal recessive form of Alport syndrome requires both copies of the faulty gene.
- Autosomal Dominant Alport syndrome (ADAS): This form occurs when any one parent passes the abnormal gene to the child.
Symptoms1,2,3,4
The symptoms associated with Alport Syndrome may vary according to the different types. Some major symptoms include:
- Blood in urine (microscopic hematuria)
- Protein in urine (proteinuria)
- Chronic kidney disease or kidney failure
- Hearing loss
- Vision abnormalities
Chronic kidney disease (CKD) emerges when kidney function begins to deteriorate. Major CKD symptoms don’t appear until they are near renal failure.
Kidney failure symptoms include:
- Swelling (edema), especially around your hands or ankles
- Extreme tiredness (fatigue)
- Nausea and vomiting
- Muscle cramps
Diagnosis1,2,3,4
To diagnose Alport syndrome, it is necessary to monitor various symptoms, indicators, and family history. There are various tests that can be used to diagnose Alport Syndrome:
- Urine & Blood Test
- Glomerular filtration rate (eGFR) Test to understand the functioning of the kidney
- Kidney Biopsy
- Hearing Test
- Vision Test
- Genetic Test
Epidemiology5
Alport syndrome is a rare condition whose frequency is unknown; however, according to the Alport syndrome news report, it is reported that the condition affects 1 in every 50,000 live births globally. According to the estimates, it affects 1 in 5,000 persons in the United States and 1 in 100,000 to 1 in 11,000 people in Europe
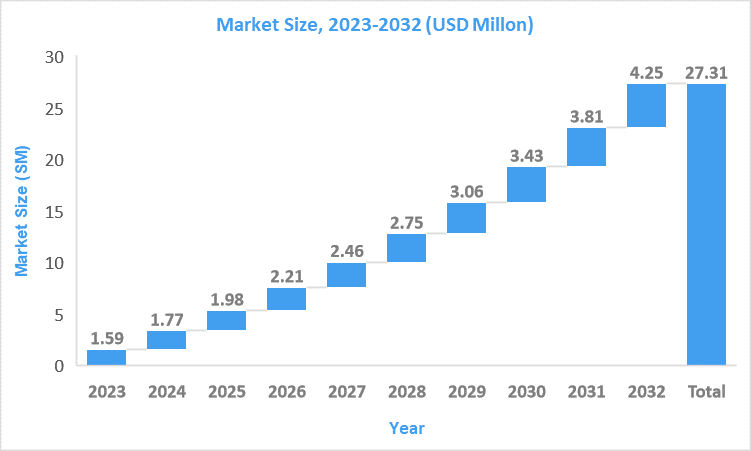
Market Size6
Management and Treatment1,2,3,4
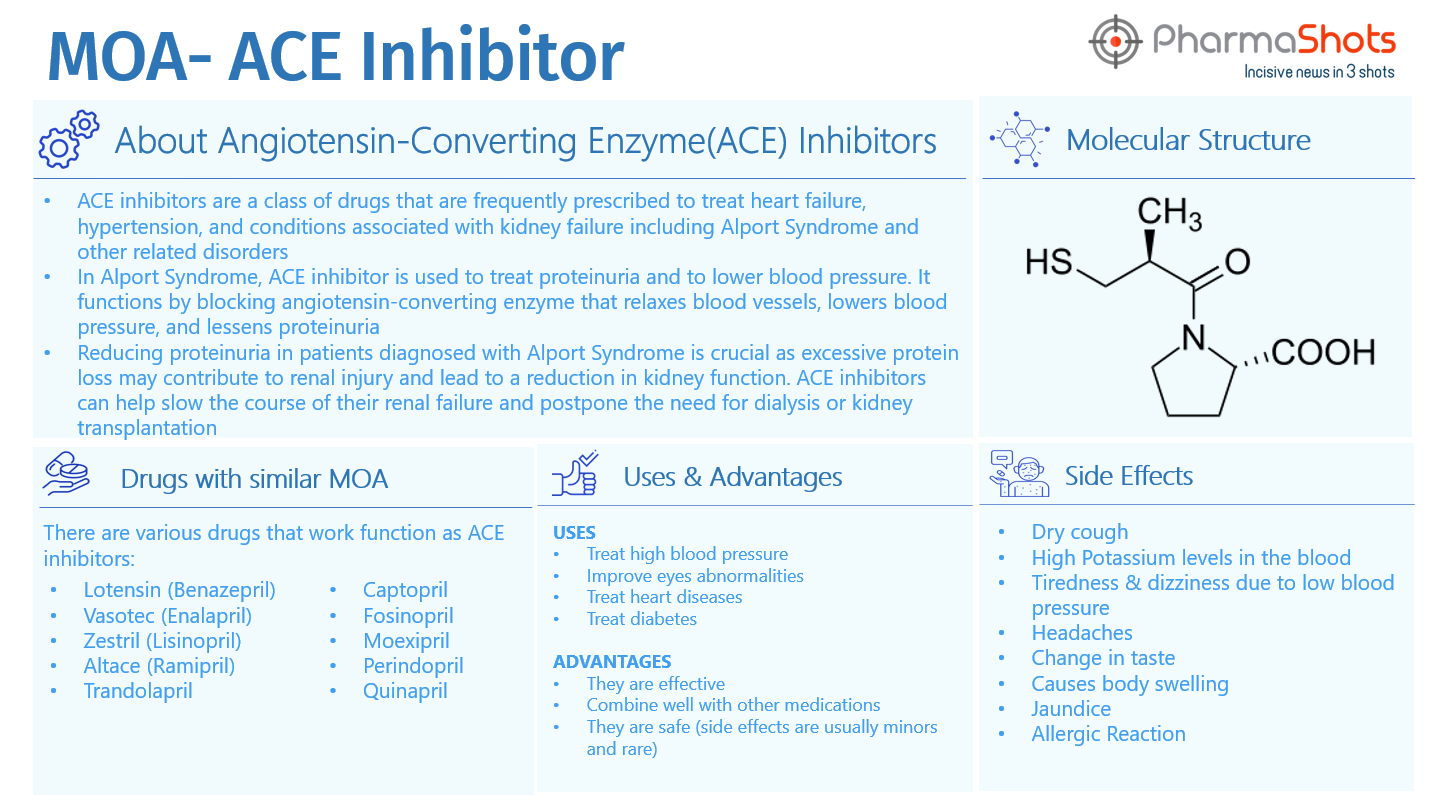
Currently, there is no treatment available for Alport Syndrome. Certain medications may help postpone renal failure, slow down renal deterioration, and control blood pressure and proteinuria, dietary modifications. Such as:
- Angiotensin-converting enzyme (ACE) inhibitors- help lower blood pressure, decrease protein levels in urine and help protect kidneys
- Angiotensin II receptor blockers (ARBs)
- Sodium-glucose transported type 2 (SGLT-2) inhibitors- help decrease risk of kidney failure
- Sodium-controlled diet- Limiting salt and sodium intake in diet help lower blood pressure and maintain kidney and heart health
Kidney transplants are often treated as one of the options for the treatment of Alport syndrome, but it won’t help with other symptoms (hearing loss and vision abnormalities).
Molecule Class Dashboard
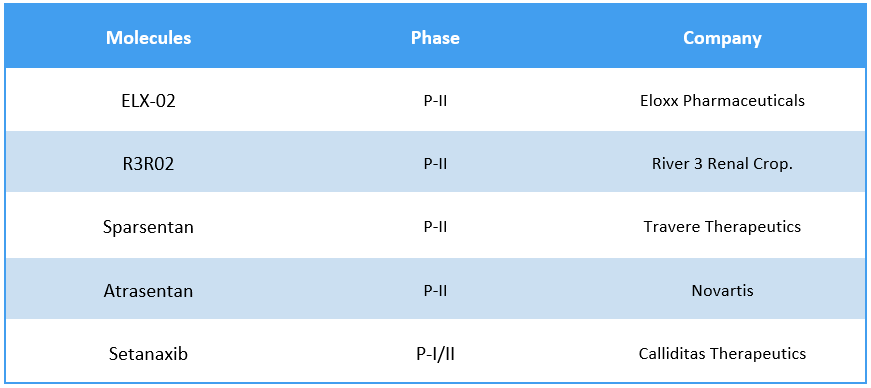
Potential Pipeline Drugs9
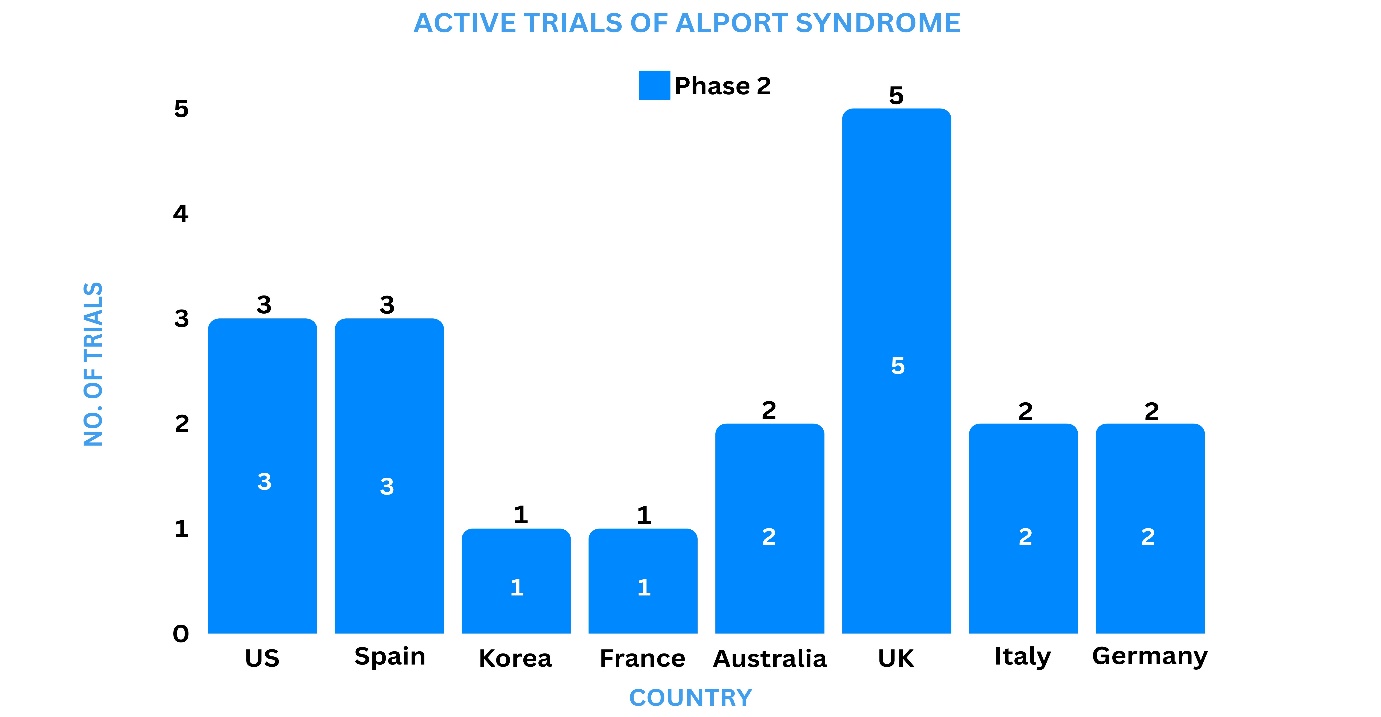
Clinical Trial Analysis9
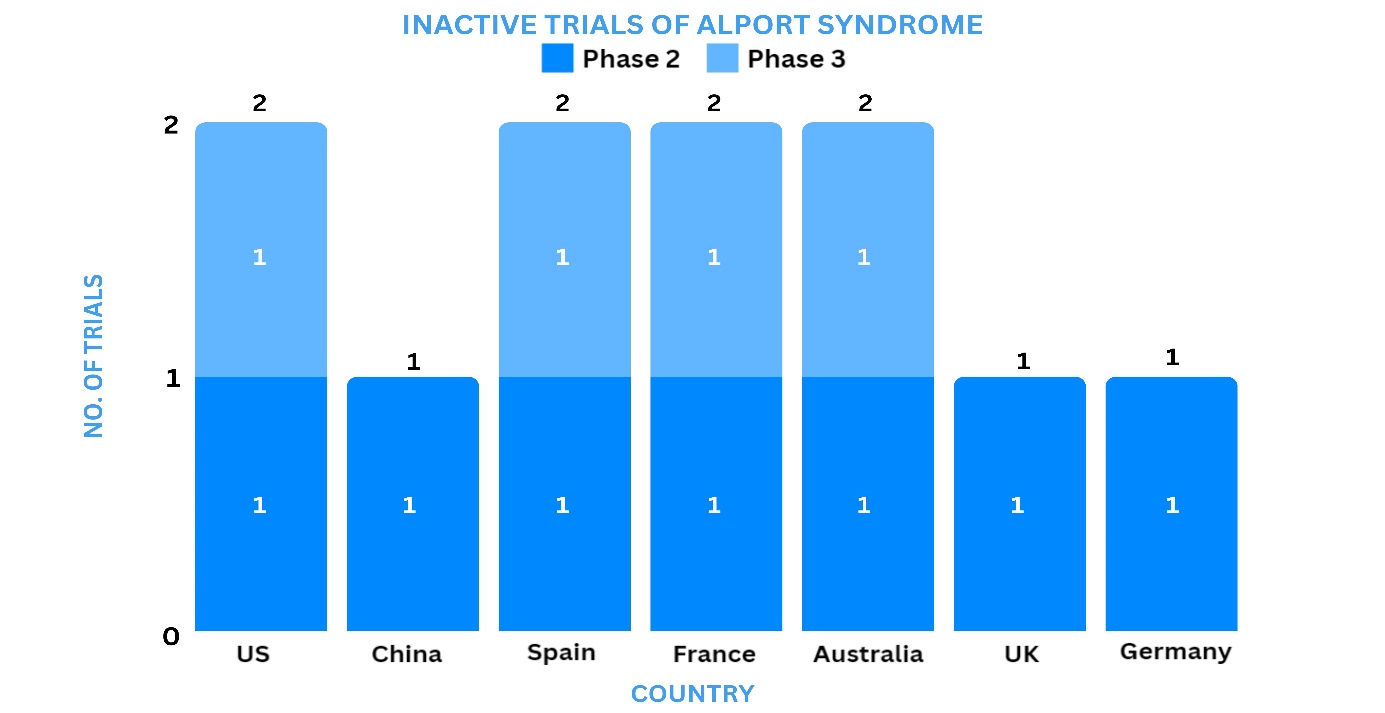
Several biopharmaceutical companies are working steadfastly to develop new treatment options for Alport Syndrome, either as monotherapy or in combination. As of March 7, 2024, about 7 clinical trials are registered worldwide for Alport Syndrome.
Some of the key molecules involved in the trials include ELX-02 (Eloxx Pharmaceuticals), R3R02 (River 3 Renal Crop.), Sparsentan (Travere Therapeutics), etc.
Based on the geographical distribution, the interventional and industry-sponsored clinical trials are classified in the below-mentioned graph into two groups based on their status: active (recruiting, active, not recruiting, not yet recruiting and enrolling by invitation, suspended) and inactive (withdrawn, terminated, and trials with unknown status)
The maximum number of active trials is being conducted in the UK, US, Spain, Australia, Italy, and Germany while relatively fewer trials were reported in Korea and France.
Noteworthy Attempt10,11,12
- In April 2021, based on the results of the P-III CARDINAL trial, Reata Pharmaceuticals submitted a new drug application for bardoxolone for the treatment of chronic kidney disease caused by Alport syndrome
- The US FDA rejected the NDA and issued a complete response letter, concluding that the submitted data did not demonstrate bardoxolone's ability to slow the loss of kidney function in patients with Alport syndrome and reduce the risk of progression to kidney failure
- On Nov 22, 2022, Reata withdrew its European application for the marketing authorization of Imbarkyd (bardoxolone) for the treatment of chronic kidney disease caused by Alport syndrome in adults and children > 12 yrs
- In May 2018, the EMA granted Imbarkyd orphan drug designation for chronic kidney disease caused by Alport syndrome
- Kyowa Kirin, Reata's Japan partner, paused the development of RTA 402 for the treatment of chronic kidney disease caused by Alport syndrome due to the lack of improvement trend in ESRD. The time of ESRD onset seems to be a critical additional factor
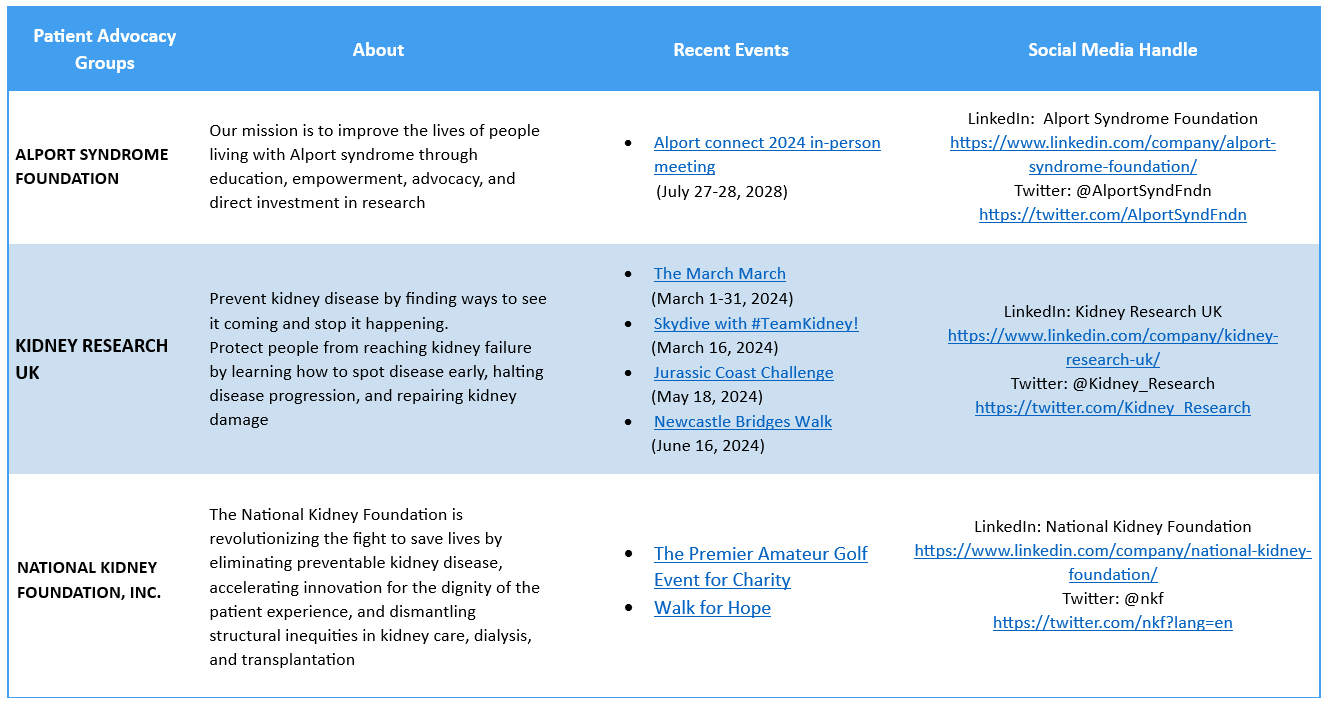
Patient Advocacy Groups (PAGs) for Alport Syndrome
References
- National Kidney Foundation
- Cleveland Clinic
- Kidney Research UK
- Alport Syndrome Foundation
- Alport Syndrome News
- Gii Research
- Mayo Clinic
- Cleveland Clinic
- ClinicalTrials.gov
- US Rejection
- EMA Withdrawal
- Japan Withdrawal
Related Post: Disease of the Month- Neuroblastoma
Tags

Disha is a content writer at PharmaShots. She is passionate and curious about recent updates and developments in MedTech and Pharma industry. She covers news related to clinical trial results and updates. She can be contacted at connect@pharmashots.com.













